Who We Are
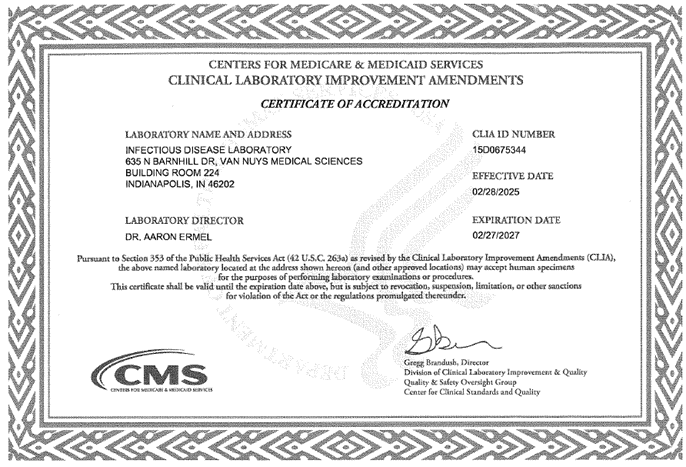
The Infectious Diseases Laboratory (IDL) has over 30 years of experience in performing diagnostic molecular testing for the detection of sexually transmitted disease pathogens. This includes a long-standing partnership with a local health department to provide testing for their sexually transmitted infection (STI) control efforts. In addition, the IDL is a designated service core laboratory for the Indiana Clinical and Translational Sciences Institute (CTSI). With over 40 years of experience in the field of clinical research, the IDL is highly skilled in performing studies that include the evaluation of new technologies for the diagnosis of infectious disease pathogens, the development of novel assays to meet the needs of individual research collaborators and tailoring our services to successfully meet study objectives. We currently provide support to numerous investigators with federal and locally funded research grants and clinical research studies with our industry sponsors. We have been continuously certified by CLIA and accredited by CAP to perform clinical testing since the inception of the laboratory back in 1985.
Our Core Competency
Elements that make us uniquely positioned for success.
- Have met stringent quality standards and personnel requirements to maintain accreditation and licensure for routine clinical diagnostic testing.
- Expertise and experience in executing both large- and small-scale studies with a proven record of meeting study objectives and timelines.
- Use the most advanced technology to perform testing for sexually transmitted and respiratory disease pathogens and many bloodborne viral pathogens.
- Ability to develop and implement new assays to meet individual study goals and needs.
- Proficient in the evaluation and validation of new instrumentation, assays, and collection methodologies.
Our Technology
We strive to maintain cutting edge technologies.
- Operate the latest automated clinical analyzers from major diagnostic manufacturers for molecular testing
- State of the art robotic platforms for large scale sample preparation and nucleic acid extractions